At Constant Temperature Which 10 Milliliter Sample
As the volume of a 1-mole sample of gas increases with temperature remaining constant the pressure exerted by the gas 1 decreases 2 increases 3 remains the same 10. Temperature constant.

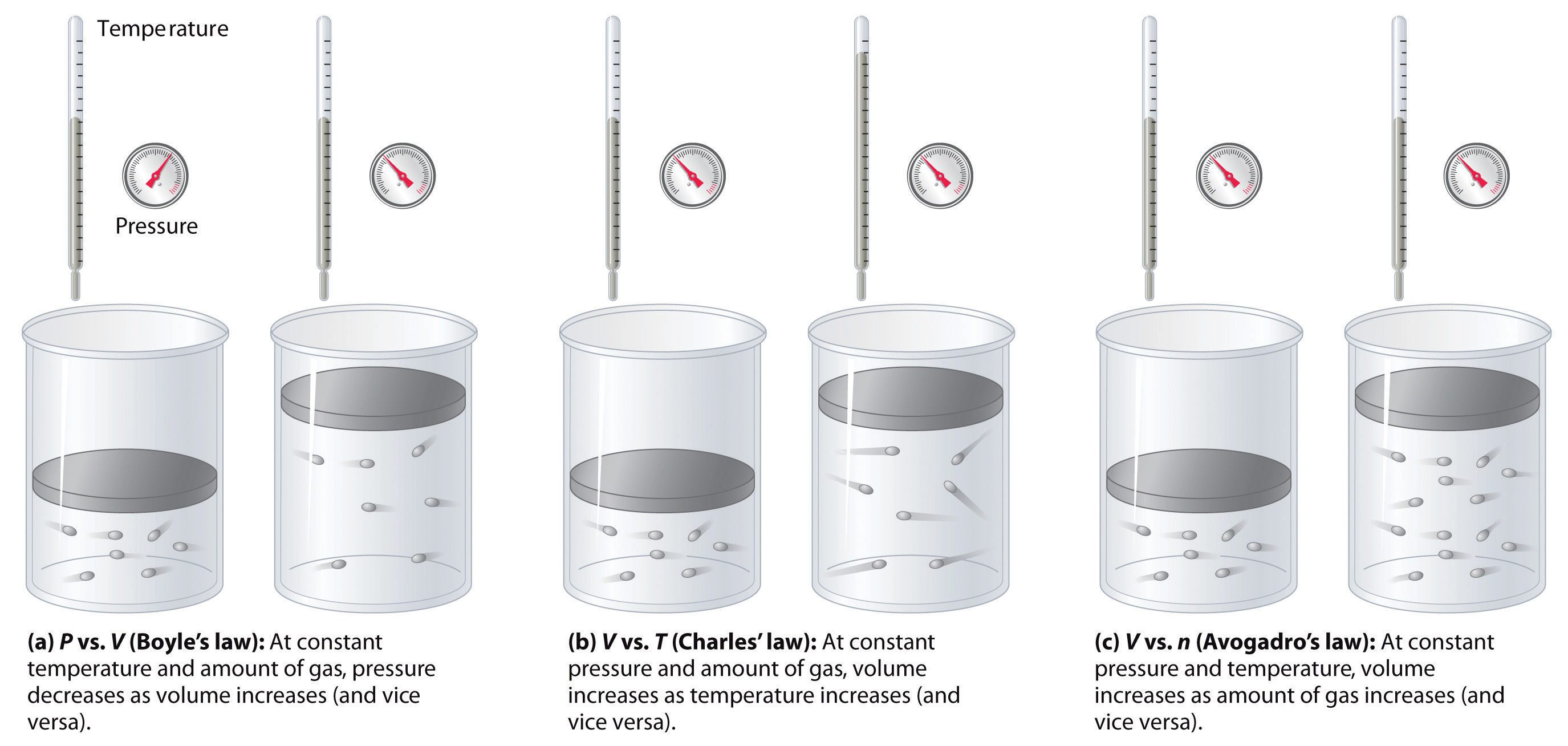
Relating Pressure Volume Amount And Temperature The Ideal Gas Law Chemistry For Majors
K under constant.
. Calculate the volume of this gas under the new conditions. A sample of oxygen that occupies 100 x 10 mL at 575 mm Hg is subjected to a pressure of 125 atm. At constant pressure the new volume of the gas will be A 0177 atm B 443 atm C 564 atm D 141 atm 42A sample of helium gas has a volume of 900.
A sample of gases contains 10 ml CH420 ml C2H2 and 30 ml CO2 at constant temperature and pressure What is the average molecular mass os sample. At constant temperature the mass of the sample A300 mL B400 mL C600 mL D100 mL 7The pressure on a 200-milliliter sample of CO2g at constant temperature is increased from 60 kPa to 120 kPa. Using the Boyles law equation the final ie new volume of the gas can be obtained as follow.
Oct 22 2011. Jan 25 2016 The. At constant temperature which 10-milliliter sample measured at STP will uniformly take the shape and volume of a 100-milliliter container into which it is placed.
At constant temperature which 10-milliliter sample measured at STP will uniformly take the shape and volume of a 100-milliliter container into what it is placed. Milliliters and a pressure of 250 atm at 298 K. The final pressure is 110 atm.
If the pressure on a 104-L sample of gas is doubled at constant temperature 0309. The correct answer to the question is Option A. A40 mlB80 mlC5 ml D10 ml 8The pressure on 20 milliliters of a gas at constant temperature is changed from 4.
If the temperature of a 500 mL sample of a gas is changed from 200. A balloon has a volume of 1751- at a temperature of 25 oc. ColorblueP_1V_1 P_2V_2 where P_1 V_1 - the pressure and.
Agas has a volume of 500 rnL at a. Final volume V₂. If the final volume of the argon sample was 182 L182 L what was the initial volume of the argon sample.
At constant temperature which 10 milliliter sample measured at stp will uniformly. Mathematically this is written as. A sample of gases contains 10 ml CH420 ml C2H2 and 30 ml CO2 at constant temperature and pressure What is the average molecular mass of sample.
Indicate the correct answer with an appropriate unit A gas sample has a volume of 250 milliliters at a temperature. If the temperature of a 105 mL sample of N₂ gas is increased from 250 C to 750 C as its pressure remains constant what will be its new volume in milliliters. In simple terms when pressure increases volume decreases and when pressure decreases volume Increases.
The pressure of a sample of argon gas was increased from 181 atm181 atm to 665 atm665 atm at constant temperature. The volume of a sample of a fixed amount of gas is decreased from 20 L. Write the correct formula.
When extended a bike pump has a volume o temperature is constant what is the new pressure in torr if the volume changes to 2225 m3. If the pressure on 360 milliliters of a gas at STP is changed to a pressure of 253 kPa at constant temperature the new volume of the gas is 1 900 ml 2 126 ml. When number of moles and temperature are kept constant pressure and volume have an inverse relationship - this is known as Boyles Law.
Is the volume decreasing more rapidly at the. The temperature remains constant. If the temperature of a 105 mL sample of N₂ gas is increased from 250 C to 750 C as its pressure remains constant what will be its new volume in milliliters.
What is the final pressure. If the gas is cooled at constant pressure until its volume is 501 mL the temperature of the gas sample will be. Chemistry Gases Molar Volume of a Gas.
What is the new pressure when the temperature is changed to 336 K and the volume is decreased to 450. A 0063 atm B 012 atm C 075 atm D 30 atm E 038 atm. A sample of hydrogen gas has a volume of 100 liters at 950 kPa and 298 K.
Chemistry 22062019 09. A 374-mL sample of H 2 at STP would contain how many grams of hydrogen. V₂ 12000 120.
When a sample of gas is compressed at a constant temperature the product of the pressure and the volume remains constant. V₂ 100 mL. What is the pressure in the expanded gas sample at constant.
CH4 and He gases is exploded by an electric discharge at room temperature with excess of. At constant pressure what temperature must be reached to increase a 100-milliliter sample of a gas initially at 300 K to a volume of 200 milliliters. Divide both side by 120.
60 200 120 V₂. 1 Answer Ernest Z. Up to 24 cash back 9.
If the pressure on a 210 times 104 mathrmmL sample of gas is doubled at constant temperature what will be the new volume of the gas. 2 Get Other questions on the subject. What is the new volume of the gas.
What will the final volume of the sample be if the temperature is held constant. Therefore the new volume of the gas is 100 mL. If the pressure of the sample is held constant and the temperature is raised to 600 K the new volume of the sample will be.
A sample of gas is in a container at low pressure and its steadily compressed at constant temperature for 10 minutes. A 251-mL sample of a gas at STP is heated to 45C. Aevaporation of 1 mol of CCl4l Bcompressing 1 mol of Ne at constant temperature from 05 atm to 15 atm Cmixing 5 mL of ethanol with 25 mL of water Draising the temperature of 100 chemistry a 104 l sample of gas at 759 mm hg pressure is expanded until its volume is 224 l.
Increases and the molecular mass remains the same. Question 10 of 40 If the temperature of a 230 mL sample of N gas is increased from 250 C to 750 C as its pressure remains constant what will be its new volume in milliliters. Asked by vishakhachandan026 15th May 2019 1037.
If temperature remains constant what is the volume of the sample at 1013 kPa. 12000 120 V₂. Question 10 of 40 If the temperature of a 230 mL sample of N gas is increased from 250 C to 750 C as its pressure remains constant what.

Kimsun Slim 2 Tpd2 Compliant Vape Kit With A 2ml Disposable Pod And A 1100mah Constant Temperature Rechargeable Battery Vape Personal Vaporizer Battery Mod
Relating Pressure Volume Amount And Temperature The Ideal Gas Law Chemistry For Majors

At Constant Temperature The Pressure Of V Ml Of A Dry Gas Was Increased From 1 Atm To 2 Atm The New Volume Will Be

Warm Up Problem What Volume Of M Solution Of Koh Is Needed To Titrate 30 0 Ml Of M H2so4 Ppt Download

Boyle S Law At A Constant Temperature The Volume Of A Sample Of Gas Varies Inversely With The Pressure Exerted On It Mathematically P 1 V 1 P Ppt Download

The Gas Laws Statements Formulae Solved Problems

Relating Pressure Volume Amount And Temperature The Ideal Gas Law Chemistry For Majors

Relating Pressure Volume Amount And Temperature The Ideal Gas Law Chemistry For Majors
The Gas Laws A Boyle S Law Boyle S Law States If The Temperature Of A Gas Sample Is Kept Constant The Volume Of The Sample Will Vary Inversely As The Pressure Varies This Statement Means That If The Pressure Increases The Volume Will Decrease If The
The Gas Laws A Boyle S Law Boyle S Law States If The Temperature Of A Gas Sample Is Kept Constant The Volume Of The Sample Will Vary Inversely As The Pressure Varies This Statement Means That If The Pressure Increases The Volume Will Decrease If The
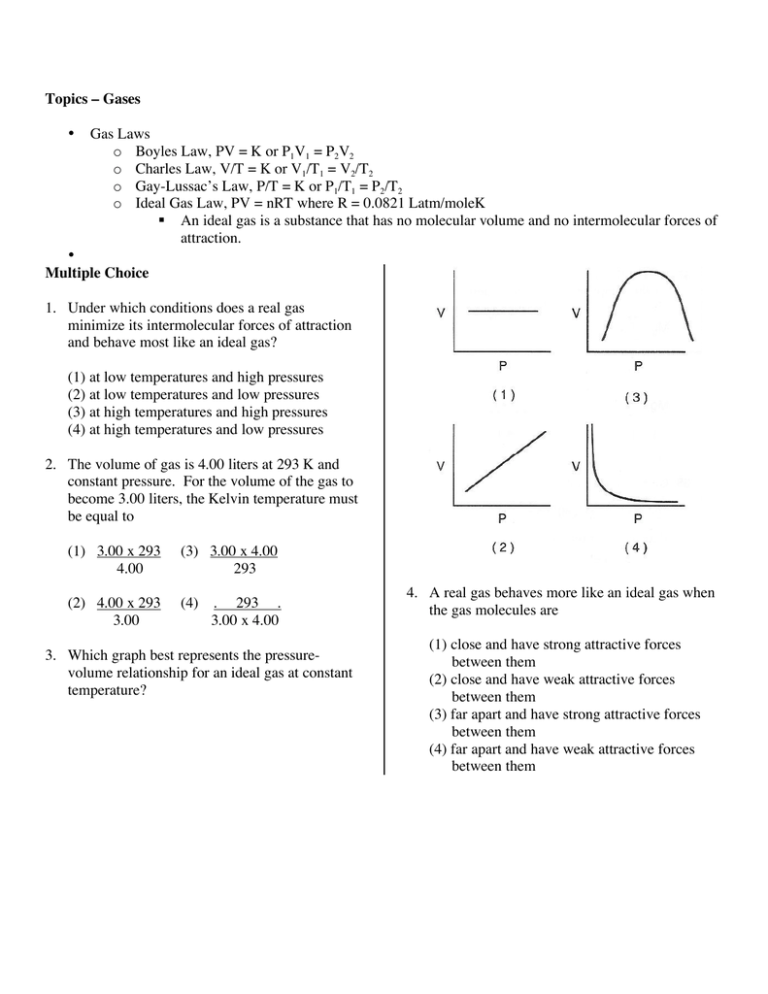
Topics Gases Gas Laws O Boyles Law Pv K Or P 1v1 P2v2 O

Part 2 The Gas Laws C L I C K T O R E T U R N Ppt Download

Chapter 2a Pure Substances Phase Change Properties Updated 9 20 09
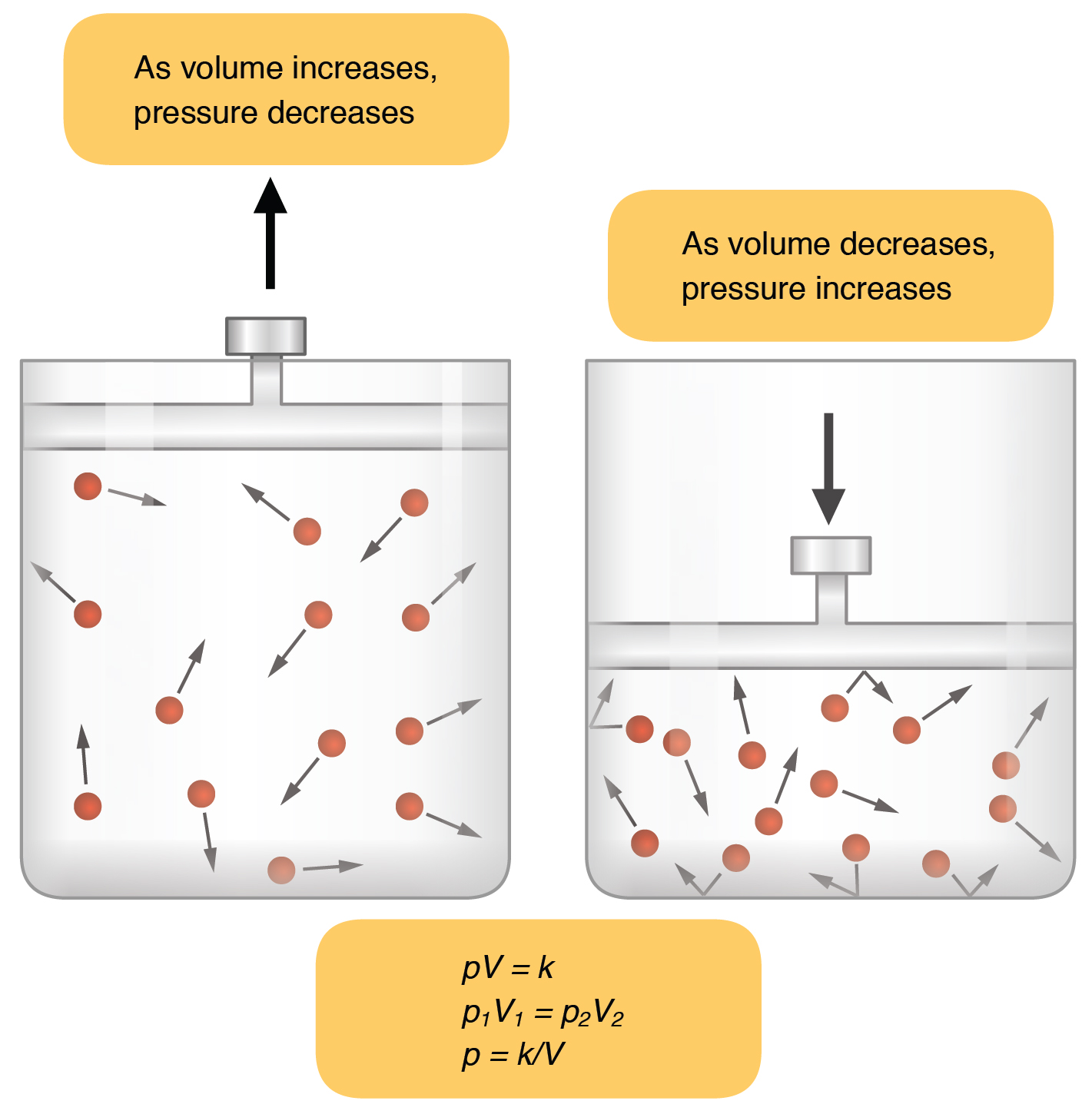
A Sample Of A Gas Has A Volume Of 2 0 Liters At A Pressure Of 1 0 Atmosphere What Will The Pressure Be When The Volume Increases To 4 0 Liters At Constant Temperature Socratic
Comments
Post a Comment